Abstract
Introduction: Chronic lymphocytic leukemia (CLL) is the most common type of leukemia among adults in the United States (US). First-line (1L) treatment includes targeted treatments like the Bruton's tyrosine kinase inhibitor ibrutinib. While treatment with ibrutinib can result in durable responses and improved survival, patients who progress while on ibrutinib have been shown to have a poor prognosis. However, there is currently limited literature describing the economic burden associated with ibrutinib failure. Therefore, this study was conducted to compare the healthcare resource utilization (HRU) and costs between patients with and without relapse/refractoriness (R/R) to ibrutinib in the US.
Methods: Data from Optum Clinformatics DataMartTM (01/2007-03/2020) were used to identify adult patients with ≥2 medical encounters with a diagnosis code for CLL or small lymphocytic lymphoma (SLL) on different dates, ≥1 pharmacy claim for ibrutinib therapy as a single-agent or combination therapy (first dispensing was the index date), and ≥12 months of continuous enrollment pre-index date (baseline period). Patients with a diagnosis for mantle cell lymphoma or with evidence of anticancer therapy (antineoplastic, radiation, or cell therapy) during baseline were excluded.
Patients were categorized as R/R to ibrutinib if they received treatment with another antineoplastic after switching/discontinuing ibrutinib; all remaining patients were considered not R/R to ibrutinib (i.e., remained on ibrutinib for the whole observation period, restarted ibrutinib, or discontinued ibrutinib treatment without initiating a different antineoplastic). Patients who died on ibrutinib or within 6 months of discontinuation without subsequent antineoplastic therapy were excluded.
All-cause and CLL/SLL-related HRU and healthcare costs incurred from the index date to the earliest of death, end of data availability or insurance coverage were evaluated and then compared in the population R/R and not R/R to ibrutinib using adjusted rate ratios for HRU and adjusted cost differences for costs. P-value and 95% confidence interval (CI) were obtained from non-parametric bootstrap procedures.
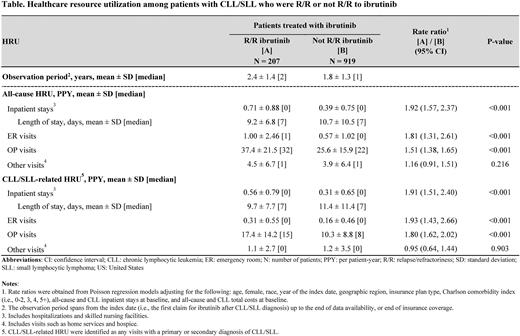
Results: Among 1,126 patients treated with ibrutinib, mean age was 72.5 years and 38.8% were female. Patients had a mean Quan-Charlson Comorbidity index of 3.4. Over 1.8 years of observation, patients treated with ibrutinib had mean (SD) 0.47 (0.80) all-cause inpatient stays, 0.67 (1.50) emergency room (ER) visits, and 28.4 (18.1) outpatient visits, resulting in $148,758 (77,099) per patient-year total all-cause healthcare costs. CLL/SLL-related HRU amounted to 0.37 (0.69) inpatient stays, 0.19 (0.48) ER visits, and 12.0 (10.8) outpatient visits, resulting in $29,079 (51,165) per patient-year CLL/SLL-related medical costs.
A total of 207 patients were R/R to ibrutinib (mean age: 73.4 years; 40.1% female) and 919 were not R/R to ibrutinib (mean age: 72.3 years; 38.5% female). Over a mean observation of 2.4 years (R/R) and 1.8 years (not R/R), patients R/R to ibrutinib had significantly more all-cause inpatient stays (rate ratio [95% CI] = 1.92 [1.57, 2.37]; P<0.001), ER visits (1.81 [1.31, 2.61]; P<0.001), and outpatient visits (1.51 [1.38, 1.65]; P<0.001) than patients not R/R to ibrutinib (Table). This led to $16,427 (95% CI: 4,627, 28,103) significantly higher incremental all-cause healthcare costs per patient-year in the R/R cohort versus not R/R (P=0.006). Similarly, rates of CLL/SLL-related inpatient stays (rate ratio [95% CI] =1.91 [1.51, 2.40]; P<0.001), ER visits (1.93 [1.43, 2.66]; P<0.001), and outpatient visits (1.80 [1.62, 2.02]; P<0.001) were significantly higher among patients R/R to ibrutinib versus not R/R (Table). This resulted in $38,252 (95% CI: 30,505, 48,023) significantly higher incremental CLL/SLL-related medical costs per patient-year in the R/R cohort versus not R/R (P<0.001).
Conclusions: In this US real-world study, patients with CLL who were treated with ibrutinib incurred substantial HRU and costs. This burden was shown to be even larger for patients who were R/R to ibrutinib, with CLL/SLL-related healthcare resource use being nearly twice higher for R/R compared to non-R/R patients. These findings highlight the unmet treatment need for patients with CLL who are R/R to ibrutinib.
Disclosures
Yang:Merck & Co., Inc.: Current Employment. Zanardo:Analysis Group, Inc.: Other: EZ is an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Merck & Co., Inc., which funded the development and conduct of this study. Lejeune:Groupe d'analyse, Ltée: Other: DL is an employee of Groupe d'analyse, Ltée, a consulting company that has provided paid consulting services to Merck & Co., Inc., which funded the development and conduct of this study. De Nigris:Merck Sharp & Dohme: Current Employment. Sarpong:Merck & Co., Inc.: Current Employment. Farooqui:Merck & Co., Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Laliberté:GSK: Research Funding; Groupe d'analyse, Ltée: Other: FL is an employee of Groupe d'analyse, Ltée, a consulting company that has provided paid consulting services to Merck & Co., Inc., which funded the development and conduct of this study.
Author notes
Asterisk with author names denotes non-ASH members.